Technologies
When will the COVID vaccine be available for kids under 5?
Pfizer asks the FDA to authorize its vaccine for kids ages 6 months to 5 years. Here’s what we know about the FDA timeline, boosters for older kids and more.
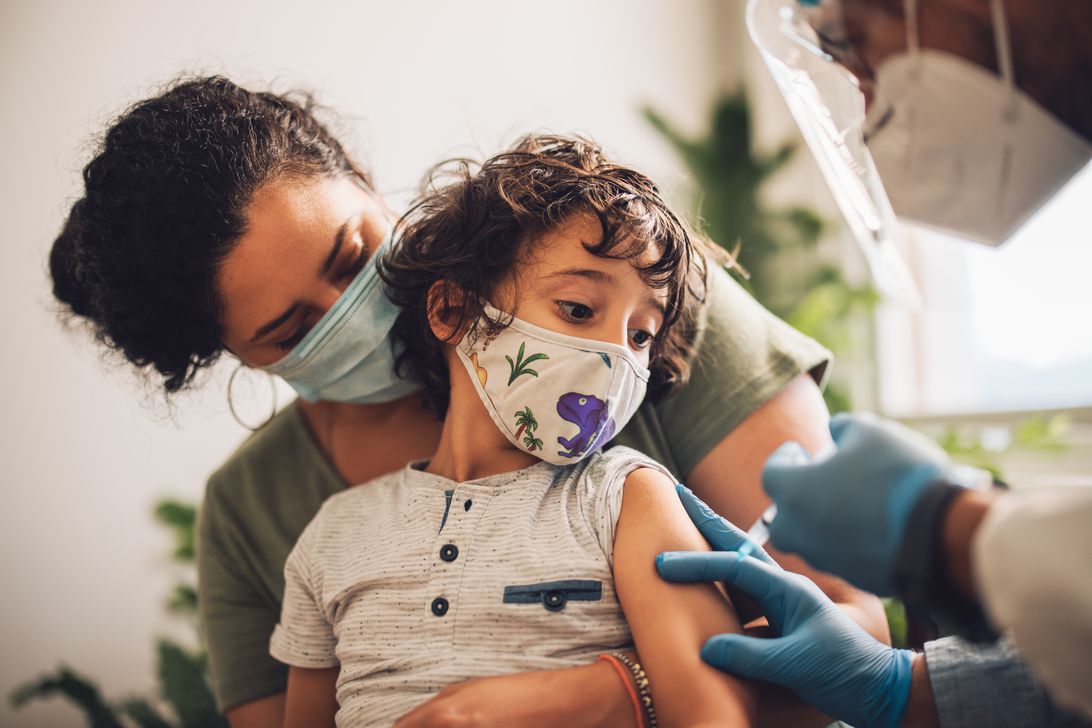
Pfizer and BioNTech on Tuesday submitted data to the US Food and Drug Administration for authorization of their COVID-19 vaccine for children 6 months through 4 years old. While the vaccine for children under 5 is expected to be a three-dose vaccine series (it’s one-tenth the volume of Pfizer’s vaccine for people 12 and up), the FDA asked the companies to submit data on the first two doses as part of a «rolling submission» process.
«Having a safe and effective vaccine available for children in this age group is a priority for the agency and we’re committed to a timely review of the data, which the agency asked Pfizer to submit in light of the recent omicron surge,» Dr. Janet Woodcock, FDA commissioner, said in a statement Tuesday. «Furthermore, children are not small adults. Because they’re still growing and developing, it’s critical that these vaccines are evaluated in well-designed and well-conducted clinical trials.»
In December, Pfizer announced that while two doses of the vaccine were effective in children ages 6 months to 2 years, two shots failed to promote a strong enough immune response in children ages 2 through 4 years. This prompted the company to start testing a three-dose version of the vaccine for children under 5. In the coming months, that data will also be submitted to the FDA for authorization, and is expected to complete the vaccine series.
Kids as young as 5 have been able to be vaccinated against COVID-19 since October — as of Dec. 19, more than 8.7 million vaccine doses have gone out to kids 5 to 11. And kids as young as 12 are eligible for booster shots, after they were recommended by the US Centers for Disease Control and Prevention in January. As the COVID-19 landscape continues to evolve, here’s what we know about COVID-19 vaccines for kids and teens. Plus, learn about the possibility of a fourth booster shot, how to find an at-home COVID-19 test and the possibility of a vaccine that works against all coronavirus variants.
When can babies and children under 5 get the vaccine?
Now that Pfizer has submitted data to the FDA, a meeting of experts that gathers to discuss safety and effectiveness data and vote on whether or not the FDA should authorize a vaccine is scheduled for Feb. 15. (The meeting is open to the public and you can tune in here or on the agency’s YouTube channel.)
If the FDA does authorize Pfizer’s vaccine for children as young as 6 months old, the CDC typically goes through the same process: An outside panel of health experts will discuss the benefits and risks of recommending the vaccine to children under 5 years old. If they vote to recommend Pfizer’s vaccine for the younger age group, the CDC’s director will likely accept the panel’s decision and the small doses of Pfizer’s COVID-19 vaccine for kids will become available in pediatricians’ offices or other clinics.
The CDC is expected to move quickly, assuming the FDA does authorize the first two doses of the anticipated three-dose vaccine series. If research finds a third dose is necessary for children under 5, it will be authorized and available after children have already started the vaccine series.
As The New York Times reported, the FDA asking a company to submit its data for authorization is an unusual move, and reflects mounting concerns about the high number of COVID-19 cases brought on by the omicron variant.
How is Pfizer’s child vaccine for babies and young kids different?
Pfizer and BioNTech’s vaccine for children 6 months through 4 years comes in two doses that are one-tenth the volume of the vaccine for people age 12 and up. A third 3-microgram dose is being researched right now and may be authorized in the near future.
The vaccine for kids 5 to 11 is one-third the dose given to everyone 12 and up, and it’s delivered in two doses. Pfizer’s vaccine for kids can also be stored for up to 10 weeks in a fridge, making it easier to administer, and the cap on the vial is orange instead of purple and gray to avoid mix-ups.
And if it helps to put your kids at ease, the needle used to administer the child’s dose of vaccine is also smaller.
For more information about Pfizer’s vaccine for children ages 5 to 11, check out this fact sheet by the FDA.
Can my child get a COVID booster?
Children as young as 12 can now get a third dose of Pfizer’s COVID-19 vaccine, given at least five months after their second shot.
Most kids younger than 12 can’t get a booster, although the CDC recommends a third dose of the Pfizer vaccine for children 5 and up who are immunocompromised. They’re eligible for a booster 28 days after their second dose.
Where can my kid get a booster shot?
Since only Pfizer’s COVID-19 vaccine is currently approved for anyone under 18, it’s generally only available in doctor’s offices and public health clinics, not pharmacies and other mass vaccination sites.
Call your pediatrician or local health clinic for a recommendation on where to go. Parents may also text their ZIP code to 438829 or use this vaccine finder link to find a clinic near them that has the child vaccine available.
Do kids really need a COVID-19 vaccine?
According to a report from the American Academy of Pediatrics, children made up 22.8% of reported COVID-19 cases for the week ending Jan. 27 this year. (The AAP says the definition of «child» varies by the states reporting.) For the same period, child COVID-19 cases were «triple the peak level» during the delta surge in 2021, the AAP reported.
While it’s true children are much less likely to get severely sick from the virus than adults, some children have died or been hospitalized with the virus. An infection, even a mild case, requires quarantining and potentially sending classmates out of the classroom and back to remote learning. And kids can experience dangerous complications from COVID-19, including long COVID and MIS-C.
There are also racial disparities in the severity of how sick children get from COVID-19: Kids ages 5 to 11 who are Black, Native American or Hispanic are three times more likely to be hospitalized with COVID-19 than white children, according to an FDA advisory panel presentation. Of that group, about one in three will require admission to the ICU.
Are booster shots safe for children?
In a statement following its authorization of booster doses for kids 12 to 15, the FDA said it reviewed real-world data from more than 6,300 children in Israel, ages 12 to 15, who received a booster shot at least five months after their second dose of Pfizer.
No additional safety concerns were reported to date in those individuals, according to the FDA.
«These additional data enabled the FDA to reassess the benefits and risks of the use of a booster in the younger adolescent population in the setting of the current surge in COVID-19 cases,» the agency said. «The data shows there are no new safety concerns following a booster in this population.»
What are the side effects? Is the COVID vaccine safe for kids?
Vaccine side effects in kids ages 5 to 11 are mostly mild and similar to those adults may experience, according to the CDC, including soreness at the injection site, fever, muscle soreness, nausea and fatigue. In a Dec. 13 report from the agency, the CDC reviewed reports from safety monitoring systems on more than 8 million doses of Pfizer’s vaccine given to kids ages 5 to 11, confirming that children’s immune systems respond well to the vaccine with common mild side effects, and that serious adverse events are rarely reported.
Inflammation of the heart muscle, known as myocarditis, and of the muscle’s outer lining, called pericarditis, are rare and typically mild side effects linked to the Moderna and Pfizer vaccines, mostly in adolescent males and young men ages 12 to 29. (Myocarditis can also occur after infection with COVID-19.)
In one study, the CDC said that 54 recipients out of a million males ages 12 to 17 experienced myocarditis following the second dose of Pfizer-BioNTech’s Comirnaty vaccine. In contrast, kids ages 5 to 11 who catch COVID-19 have a higher risk of multisystem inflammatory syndrome, or MIS-C, a potentially serious complication involving inflammation of the heart, lungs, kidneys, brain, skin, eyes or other organs.
«The bottom line is that getting COVID is much riskier to the heart than anything in this vaccine, no matter what age or sex you are,» Dr. Matthew Oster, a pediatric cardiologist at Children’s Healthcare of Atlanta, told the CDC in November, ABC News reported.
Do I need to give consent for my young child to get vaccinated?
Yes, parents generally need to consent to their children receiving medical care, including Pfizer’s COVID-19 vaccine. This is especially true for younger children.
However, depending on which state you live in, there may be a legal precedent for teens and other kids to request the vaccine without your permission: Tennessee’s vaccine director, Michelle Fiscus, was fired in August allegedly in part for sending out a memo detailing Tennessee’s «mature minor doctrine,» which explains how minors may seek medical care without the consent of their parents.
If my child has a serious health condition, can they get a third shot?
The CDC recommended a third dose for children as young as 5 who are «moderately to severely» immunocompromised, 28 days after their second shot. This guidance for immunocompromised children (including kids who’ve had an organ transplant or are taking medications that suppress the immune system) is in line with guidance for adults whose bodies don’t mount a good immune response to the COVID-19 vaccines.
My child has allergies. Can they get the vaccine?
Yes, though you might be asked to stick around the waiting room so health care providers can monitor them for (extremely rare) allergic reactions that can occur after any vaccination.
«If the child has a history of anaphylaxis or other severe allergies, then the observation time after the injection may be 30 minutes instead of 15,» said Dr. Anne Liu, an infectious disease specialist with Stanford Hospital and Clinics and the Lucile Packard Children’s Hospital. Children who have been prescribed an EpiPen for any reason should bring it to their vaccine appointment, Liu added.
As with adults, children with an allergy to an ingredient in Pfizer’s COVID-19 shouldn’t take it. You can find a list of ingredients in Pfizer’s vaccine for kids ages 5 to 11 on the FDA’s fact sheet.
Can my child get the COVID-19 shot at the same time as other vaccines?
According to the CDC, your child may get other vaccines when they go in for their COVID shot without waiting 14 days between appointments. Flu shots can be given to children ages 6 months and older.
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.
Technologies
Today’s NYT Mini Crossword Answers for Saturday, Feb. 28
Here are the answers for The New York Times Mini Crossword for Feb. 28.

Looking for the most recent Mini Crossword answer? Click here for today’s Mini Crossword hints, as well as our daily answers and hints for The New York Times Wordle, Strands, Connections and Connections: Sports Edition puzzles.
Need some help with today’s Mini Crossword? As is usual for Saturday, it’s pretty long, and should take you longer than the normal Mini. A bunch of three-initial terms are used in this one. Read on for all the answers. And if you could use some hints and guidance for daily solving, check out our Mini Crossword tips.
If you’re looking for today’s Wordle, Connections, Connections: Sports Edition and Strands answers, you can visit CNET’s NYT puzzle hints page.
Read more: Tips and Tricks for Solving The New York Times Mini Crossword
Let’s get to those Mini Crossword clues and answers.
Mini across clues and answers
1A clue: Rock’s ___ Leppard
Answer: DEF
4A clue: Cry a river
Answer: SOB
7A clue: Clean Air Act org.
Answer: EPA
8A clue: Org. that pays the Bills?
Answer: NFL
9A clue: Nintendo console with motion sensors
Answer: WII
10A clue: ___-quoted (frequently said)
Answer: OFT
11A clue: With 13-Across, narrow gap between the underside of a house and the ground
Answer: CRAWL
13A clue: See 11-Across
Answer: SPACE
14A clue: Young lady
Answer: GAL
15A clue: Ooh and ___
Answer: AAH
17A clue: Sports org. for Scottie Scheffler
Answer: PGA
18A clue: «Hey, just an F.Y.I. …,» informally
Answer: PSA
19A clue: When doubled, nickname for singer Swift
Answer: TAY
20A clue: Socially timid
Answer: SHY
Mini down clues and answers
1D clue: Morning moisture
Answer: DEW
2D clue: «Game of Thrones» or Homer’s «Odyssey»
Answer: EPICSAGA
3D clue: Good sportsmanship
Answer: FAIRPLAY
4D clue: White mountain toppers
Answer: SNOWCAPS
5D clue: Unrestrained, as a dog at a park
Answer: OFFLEASH
6D clue: Sandwich that might be served «triple-decker»
Answer: BLT
12D clue: Common battery type
Answer: AA
14D clue: Chat___
Answer: GPT
16D clue: It’s for horses, in a classic joke punchline
Answer: HAY
Technologies
Ultrahuman Ring Pro Brings Better Battery Life, More Action and Analysis
The company’s new flagship smart ring stores more data, too. But that doesn’t really help Americans.

Sick of your smart ring’s battery not holding up? Ultrahuman’s new $479 Ring Pro smart ring, unveiled on Friday, offers up to 15 days of battery life on a single charge. The Ring Pro joins the company’s $349 Ring Air, which boosts health tracking, thanks to longer battery life, increased data storage, improved speed and accuracy and a new heart-rate sensing architecture. The ring works in conjunction with the latest Pro charging case.
Ultrahuman also launched its Jade AI, which can act as an agent based on analysis of current and historical health data. Jade can synthesize data from across the company’s products and is compatible with its Rings.
«With industry-leading hardware paired with Jade biointelligence AI, users can now take real-time actionable interventions towards their health than ever before,» said Mohit Kumar, CEO of Ultrahuman.
No US sales
That hardware isn’t available in the US, though, thanks to the ongoing ban on Ultrahuman’s Rings sales here, stemming from a patent dispute with its competitor, Oura Ring. It’s available for preorder now everywhere else and is slated to ship in March. Jade’s available globally.
Ultrahuman says the Ring Pro boosts battery life to about 15 days in Chill mode — up to 12 days in Turbo — compared to a maximum of six days for the Air. The Pro charger’s battery stores enough for another 45 days, which you top off with Qi-compatible wireless charging. In addition, the case incorporates locator technology via the app and a speaker, as well as usability features such as haptic notifications and a power LED.
The ring can also retain up to 250 days of data versus less than a week for the cheaper model. Ultrahuman redesigned the heart-rate sensor for better signal quality. An upgraded processor improves the accuracy of the local machine learning and overall speed.
It’s offered in gold, silver, black and titanium finishes, with available sizes ranging from 5 to 14.
Jade’s Deep Research Mode is the cross-ecosystem analysis feature, which aggregates data from Ring and Blood Vision and the company’s subscription services, Home and M1 CGM, to provide historical trends, offer current recommendations and flag potential issues, as well as trigger activities such as A-fib detection. Ultrahuman plans to expand its capabilities to include health-adjacent activities, such as ordering food.
Some new apps are also available for the company’s PowerPlug add-on platform, including capabilities such as tracking GLP-1 effects, snoring and respiratory analysis and migraine management tools.
Technologies
The FCC Just Approved Charter’s $34.5B Cox Purchase. Here’s What It Means for 37M Customers
-

 Technologies3 года ago
Technologies3 года agoTech Companies Need to Be Held Accountable for Security, Experts Say
-

 Technologies3 года ago
Technologies3 года agoBest Handheld Game Console in 2023
-

 Technologies3 года ago
Technologies3 года agoTighten Up Your VR Game With the Best Head Straps for Quest 2
-

 Technologies4 года ago
Technologies4 года agoBlack Friday 2021: The best deals on TVs, headphones, kitchenware, and more
-

 Technologies5 лет ago
Technologies5 лет agoGoogle to require vaccinations as Silicon Valley rethinks return-to-office policies
-

 Technologies5 лет ago
Technologies5 лет agoVerum, Wickr and Threema: next generation secured messengers
-

 Technologies4 года ago
Technologies4 года agoOlivia Harlan Dekker for Verum Messenger
-

 Technologies4 года ago
Technologies4 года agoiPhone 13 event: How to watch Apple’s big announcement tomorrow
