Technologies
COVID vaccine boosters: When is it time for that extra shot? Here’s what we know
Officials set an initial Sept. 20 date for vaccine booster shots. We’ll update you on the White House’s booster plan and tell you what’s happening with each vaccine.
On Friday, The New York Times reported that officials from the Food and Drug Administration as well as the Centers for Disease Control and Prevention are advising the White House to scale back its original booster recommendation. Last month, President Joe Biden announced that his administration would start offering boosters to those who’d been fully immunized at least eight months ago by the Pfizer-BioNTech or Moderna vaccine. The original plan was contingent on approval by the FDA, which still says there’s not enough data on booster shots to go forward.
Government officials are now cautioning that boosters may be available just for those who received the Pfizer vaccine, because regulators may need more time to evaluate the vaccines from Moderna and Johnson & Johnson.
The booster recommendations come as research shows how the effectiveness of the vaccines can decline. An additional shot from Johnson & Johnson, Pfizer or Moderna could provide enhanced protection against the COVID-19 delta variant as it surges across the country. «Recent data makes clear that protection against mild and moderate disease has decreased over time,» US Surgeon General Vivek Murthy said during an August briefing on COVID-19 vaccines. «This is likely due to both waning immunity and the strength of the widespread delta variant.»
What does all this mean for you? Read on for what we know about COVID-19 booster shots today, including who can get them now, why they’re needed, how they relate to breakthrough infections and what the controversy has been surrounding third shots. We’ll be updating this as new information is released.
Why would a Pfizer vaccine booster be authorized first?
According to a report by The New York Times on Sept. 3, administration health officials warn that the FDA and CDC may not have enough time to approve boosters from Moderna and Johnson & Johnson in time to meet Biden’s goal of starting booster shots on Sept. 20.
While the Pfizer plan seems to remain on track, Moderna announced it just began submitting booster data to the FDA, so the process may take some more time to determine a recommended dosage for a third Moderna shot. Data from Johnson & Johnson has not yet been delivered.
Who would qualify for a booster shot and when?
In August, healthofficials in the Biden administration recommended an additionalshot for Americans 18 and over who are fully vaccinated with thePfizer or Moderna shots, proposing a booster eight months after being fully vaccinated.
During a meeting with Israeli Prime Minister Naftali Bennett at the end of August, Biden said administration health officials were evaluating a five-month gap, based in part on data from Israel’s booster program. The news followed a report by The Wall Street Journal that the Biden administration was evaluating a booster shot six months after the last jab.
Waiting months in between doses allows the immune system to develop a full response before it is helped by a boost. Whatever the time gap, the booster plan would need to be evaluated and approved by the FDA. The plan is also pendingrecommendation by the CDC’sAdvisory Committee on Immunization Practices.
«We believethat that third dose will ultimately be needed to provide the fullestand continual extent of protection that we think people need from thevirus,» Murthy said. «Our plan is to stay ahead of this virus by beingprepared to offer COVID-19 booster shots to fully vaccinated adults 18years and older.» Murthy said the FDA will evaluate booster shots forthose younger than 18 years of age, and the administration will followFDA recommendations for minors.
Does full FDA approval of Pfizer’s vaccine include a booster?
On Aug. 23, the FDA approved Pfizer’s COVID-19 vaccine for two doses for people 16 and older. The Pfizer vaccine is the first to receive FDA approval, while Moderna and Johnson & Johnson vaccines are available under an emergency use authorization. And for children ages 12 to 15, Pfizer’s vaccine is authorized for emergency use.
While a third dose for some immunocompromised individuals is authorized for emergency use, the FDA final authorization does not include a booster shot, which the Biden administration is hoping to roll out as soon as this month. But some health care experts believe the two-shot approval could give a push to those who were waiting on the FDA before getting vaccinated.
«The moment you have been waiting for is here,» Biden said, following the FDA approval. «Now it has been granted. Those who’ve been waiting for full approval should go get their shot now.»
The approval could also lead to more businesses, schools and venues mandating vaccine requirements.
What about a Johnson & Johnson vaccine booster shot?
On Aug. 25, Johnson & Johnson saida booster shot of its COVID-19 vaccine increases antibody responses inthose who received the company’s one-dose vaccine, based on interimdatafrom anearly trial. The company said it would work with public healthofficials on a plan for a booster shot for eightmonths or longerafter the first dose of its vaccine.
Currently,the one-doseJohnson & Johnson vaccine is available under anemergency useauthorization for individuals 18 years of age and older.
Bidenadministration health officials said they expect those who receivedtheone-dose Johnson & Johnson vaccine will need another jab, butmoreresearch is necessary. «We expect more data on J&Jin thecomingweeks,» Murthy said when announcing plans for booster shots in late August.»With thatdata in hand, we willkeep the public informed with thetimely plan forJ&J boostershots.»
What about those eligible for booster shots now?
Some people who already are eligible under guidelines from the CDC can go out and get their third dose of a COVID-19 vaccine immediately. The list of immunocompromised people who can get a third shot includes solid-organ transplant recipients and people who have an «equivalent level of immunocompromise» and who have a reduced ability to fight off infections, making them more vulnerable to the coronavirus. Booster authorization hasn’t yet been expanded more broadly to those with other chronic medical conditions.
The current CDC recommendation is for an additional dose of the two-shot vaccine for certain immunocompromised people. Within that category, the recommendation is for those 18 and older for the Moderna vaccine, and 12 and older for the Pfizer vaccine. The FDA didn’t authorize an additional dose of the Johnson & Johnson vaccine, and because of a lack of data the CDC hasn’t recommended a second dose for immunocompromised people who got the one-shot vaccine.
About 3% of US adults are immunocompromised, according to the CDC, but research suggests they account for about 44% of hospitalized breakthrough cases of COVID-19. Not only are they more likely to get very ill from COVID-19, they also have a lower antibody response to vaccines and are at a higher risk of transmitting the virus.
Those with other conditions, like diabetes and heart disease, aren’t advised to get a booster, at least for now. Here’s a list of people the CDC recommends get an extra dose if they got the Pfizer or Moderna vaccine:
- Those with advanced or untreated HIV infection.
- Cancer patients and transplant recipients who are taking certain immunosuppressive drugs.
- Those receiving active cancer treatment for tumors or cancers of the blood.
- Those with moderate or severe primary immunodeficiency.
- Patients being treated with high-dose corticosteroids or other drugs that may suppress immune response.
- People who received a stem cell transplant within the last two years and are taking certain drugs. The CDC says to talk to your medical provider about your health condition and whether a third shot is appropriate.
If you’re unsure whether you’re qualified, the CDC says to talk to your medical provider about your health condition and whether a third dose is appropriate.
Will the booster shots be free and available?
The current one-dose vaccine shot from Johnson & Johnson and two-dose vaccines from Moderna and Pfizer are free to anyone who wants to get vaccinated. And the additional shots will be free too. «These booster shots are free,» Biden said. «It will be easy. Just show your vaccination card and you’ll get a booster. No other ID. No insurance. No state registry requirements.»
«It will be just as easy and convenient to get a booster shot as it is to get a first shot today. We have enough vaccine supply for every American,» said White House COVID-19 Response Coordinator Jeff Zients, adding that those who are eligible will be able to get a booster at roughly 80,000 places across the country, including over 40,000 local pharmacies. Zients said 90% of Americans have a vaccine site within 5 miles of where they live.
What’s behind the need for COVID-19 booster shots?
«The COVID-19 vaccines that are authorized in the United States have been remarkably effective, even against the widespread delta variant,» Murthy said. «But we know that even highly effective vaccines become less effective over time.»
Calling the eradication of the COVID-19 virus «unlikely,» a UK scientific advisory group found (PDF) that there’s a «realistic possibility» that a variant will emerge that is resistant to the current battery of vaccines. Governments, public health organizations and vaccine makers are all tracking developments in coronavirus variants like delta and lambda, hoping to determine if booster shots targeting new variants will be needed among the general population.
What’s the relationship to COVID-19 breakthrough cases?
As of July, in the US, «breakthrough» coronavirus cases caused by the dominant delta variant amount to less than 1% of people who are fully vaccinated. Both the Moderna and Pfizer vaccines have proved to be more than 90% effective against hospitalizations and death. Nonetheless, a CDC study shows that vaccinated people can both contract the highly contagious delta variant and spread it. According to a widely reported internal CDC memo, the delta variant spreads as easily as chicken pox, which is considered more contagious than the flu but less contagious than measles.
The surge in new COVID-19 cases is primarily affecting unvaccinated people and causing community spread, and in turn, prompting the return of mask mandates and guidance in hard-hit areas, even for people who have full vaccine protection. The debate over mask use and vaccine boosters underscores how scientists and other health experts continue to grapple with the uncertainties of COVID-19.
According to Murthy, «We are concerned that this pattern of decline we are seeing will continue in the months ahead, which could lead to reduced protection against severe disease, hospitalization and death.»
What’s the global controversy over booster shots?
Israel is now administering third doses of the vaccine to those 50 and older, and the UK has similar plans for September. However, this is resulting in a backlash among countries that are struggling to deliver first and second shots to residents.
World Health Organization Director-General Tedros Adhanom Ghebreyesus called for a «moratorium» on booster shots in high-income countries, citing the global disparity in vaccine distribution. Of the 4 billion doses administered globally, 80% have gone to high- and upper-middle income countries that make up less than half the world’s population, he said.
«We cannot accept countries that have already used most of the global supply of vaccines using even more of it, while the world’s most vulnerable people remain unprotected. We call on vaccine producers to prioritize Covax,» Tedros said, referring to the world’s COVID-19 vaccine distribution program.
White House Press Secretary Jen Psaki said on Aug. 17 that the US will have enough vaccines to both provide boosters for those who are fully vaccinated in the US and meet the global demand. «We have long planned from enough supply,» she said.
The US has so far shipped 115 million vaccine doses to 80 different countries, Zients said. «Our wartime efforts will continue doing everything we can to get even more people vaccinated both here at home and around the world. We can and must do both at the same time because that’s what it’s going to take to end this pandemic,» he said.
Is it possible to mix and match COVID-19 vaccines?
According to The New York Times, administration officials will recommend people get a booster of the same vaccine they originally received. The CDC now says a third dose of a different vaccine brand is permitted if a dose of the same type isn’t available.
Other global health agencies and countries are testing administered vaccines from two different manufacturers. In the UK, for example, a recent study found that those who received a first dose of the AstraZeneca vaccine and a second of Pfizer had a higher immune response than those who received two doses of the AstraZeneca vaccine.
While we watch how the situation develops, here’s what we know about the delta variant and info on whether you need to continue to wear a mask.
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.
Technologies
Google Gives Chrome an AI Side Panel and Lets Gemini Browse for You
The update also includes Nano Banana image tools and deeper integrations with Google apps like Gmail, Calendar, Maps and Flights.

Google is turning Chrome into something closer to a digital copilot.
In the next wave of Gemini updates rolling out, Google on Wednesday revealed a set of new AI-powered features coming directly to its browser, aimed at reducing the frustrations of exploring the internet each day. Built on Gemini 3, the updates introduce an always-available side panel, deeper app integrations, creative image tools and a new browser agent called auto browse that can complete multistep tasks on your behalf.
Essentially, Google wants Chrome to be like an AI wingman that browses, compares and multitasks for you.
Read more: More AI Is Coming to Google Search, Including a Chatbot-Like Interface
Now you can automate browsing
To me, the standout new addition is auto browse, a browser agent designed to handle tedious and time-consuming chores. Instead of hopping between tabs, filling out forms or manually comparing prices of things like products or flights, you can ask Chrome to do the legwork.
Auto browse can research flights and hotels across different dates, collect documents, schedule appointments, manage subscriptions and help with tasks like renewing a driver’s license or filing expense reports.
In a live demo I saw, Product Lead Charmaine D’Silva used the new tools to plan a family vacation. Gemini compared destinations and prices across multiple travel sites, checked school calendars to see when her kids were off and lined up schedules to find workable travel windows. When it came time to book, though, D’Silva emphasized that the final decision and purchase were still hers, underscoring Google’s plan to keep humans in control for key tasks like booking and purchases.
The feature is rolling out to AI Pro and Ultra subscribers in the US now, signaling Google’s broader push toward more agentic AI experiences.
Don’t miss any of our unbiased tech content and lab-based reviews. Add CNET as a preferred Google source.
A new side panel experience
Another update rolling out now is a redesigned Gemini side panel in Chrome, available across MacOS, Windows and Chromebook Plus. Instead of opening a separate tab, Gemini now lives alongside whatever you’re working on, making it easier to multitask without breaking your flow. Testers have used it to summarize reviews across sites, compare shopping options and juggle packed calendars while keeping their main task front and center.
AI image editing with Nano Banana
Chrome is also trying to become more creative. Google is bringing Nano Banana, its AI image editing and generation tool, directly into the browser. You can now edit and reimagine images you find on the web without downloading files or switching apps — whether that’s mocking up a living room redesign or turning raw data into an infographic at work.
Chrome connects with other Google apps
Under the hood, Gemini in Chrome is becoming more connected to the rest of Google’s ecosystem. Integrations with Gmail, Calendar, Maps, YouTube, Google Flights and Shopping will allow the assistant to pull in relevant context and take action across apps. Planning a trip, for example, could involve referencing an old email, checking flight options and drafting a follow-up email to your travel companions. Now all in one place.
More to come
Looking ahead, Google says personal intelligence is coming to Chrome in the coming months. With user opt-in, Gemini will remember context from past interactions to deliver more tailored, proactive help across the web, while giving you control over what data is connected and when.
Technologies
If You Drink Decaf, Read This: More Than 80,000 Keurig Pods Recalled
Here’s how to get a full refund if you bought these coffee pods.

If you’re a decaf K-Cup drinker, this message is for you. Keurig has recalled the McCafe Premium Roast Decaf Coffee K-Cup Pods because they may contain caffeine.
Here’s everything to know.
Don’t miss any of our unbiased tech content and lab-based reviews. Add CNET as a preferred Google source.
What was recalled?
Keurig Dr Pepper voluntarily recalled 960 cartons of McCafe Premium Roast Decaf Coffee K-Cup Pods, according to a US Food and Drug Administration memo. The reason listed for the recall reads: «Product is labeled as decaf, but might contain caffeine.»
CNET chose McCafé Premium Roast as the best K-Cup, although the decaffeinated version was not included. It is unclear at this time how many states sold the cartons.
A representative for Keurig Dr Pepper did not immediately respond to a request for comment.
How to know if you have a recalled product
The recalled items will have the following information:
- Best by date: 17 NOV 2026
- Batch number: 5101564894
- Material number: 5000358463
- ASIN: B07GCNDL91
- UPC: 043000073438
The recall is ongoing. If you have a recalled product, you can return it to your place of purchase for a full refund.
Technologies
The Samsung Galaxy Z TriFold’s Nearly $3,000 Price Might Unfold Your Whole Wallet
This double-folding phone will be the most expensive mainstream handset released in the US.
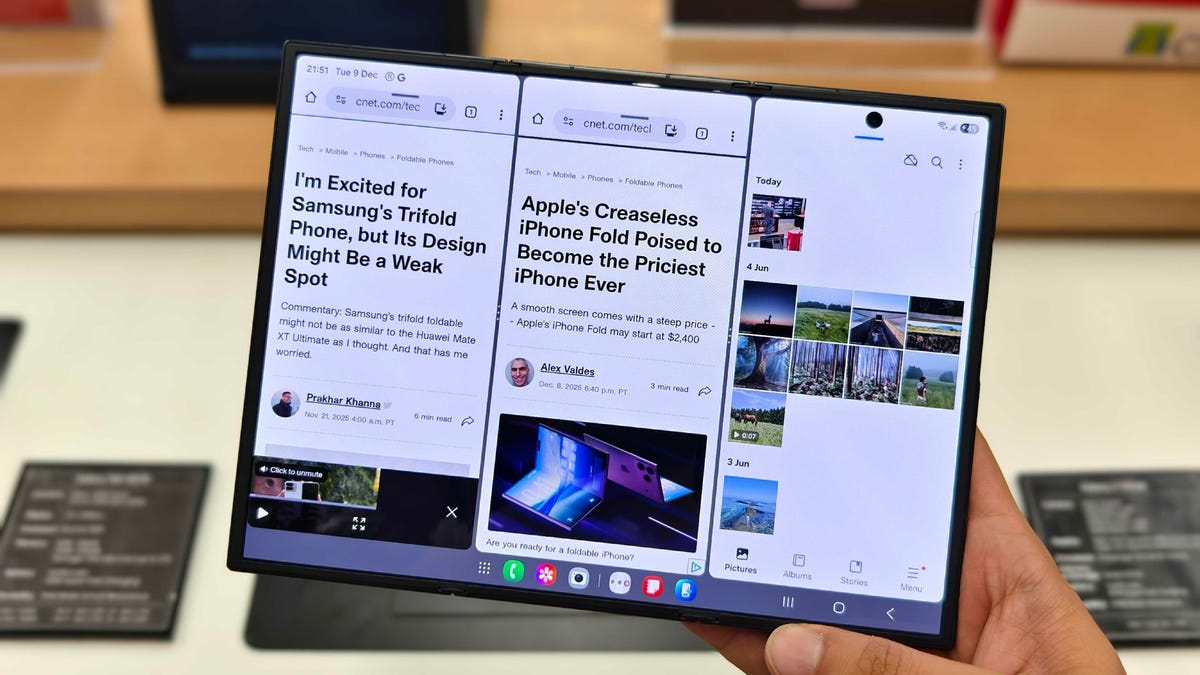
Samsung’s twin-hinged Galaxy Z TriFold is nearly on sale, coming before the Galaxy S26 launch next month. Starting Jan. 30, foldable phone fans who want the most advanced device in the US can pick one up, but they’ll have to pay a hefty price: The device starts at a jaw-dropping $2,900.
Yes, for over three times the price of a Galaxy S25, you can pick up the most advanced smartphone — and certainly the most expensive — Samsung has ever rolled out. Even the Galaxy Z Fold 7, which starts at $2,000 with 256GB of storage, only reaches $2,420 at the highest 1TB storage configuration.
As products across all industries get costlier, phone-makers have priced foldables in an even more premium tier than the most innovative flat smartphones (like the iPhone 17 Pro Max and Galaxy S25 Ultra). It seems Samsung will use the twin-hinged Galaxy Z TriFold to set an even higher price ceiling for smartphones.
Don’t miss any of our unbiased tech content and lab-based reviews. Add CNET as a preferred Google source.
Anyone who buys the Galaxy Z TriFold will get one of the most technically impressive handsets released in the US. But is the technology worth the cost?
The Galaxy Z TriFold unfolds into a 10-inch inner display that rivals the screens of full-size tablets. It’s noticeably larger than the 8-inch inner screen on the single-hinged Galaxy Z Fold 7 foldable. Its two hinges, built of titanium, are tested to endure 200,000 folds, according to Samsung.
When unfolded, the Z TriFold is 3.9mm at its thinnest point. That’s slightly outdone by the slimmer Huawei Mate XT’s 3.6mm, which beat Samsung to market by an entire year with a trifold that’s not available in the US. That might be nearing the limit for phone thinness, as it’s barely enough to accommodate the USB-C port at the bottom of either device.
The Galaxy Z TriFold and Huawei Mate XT are roughly comparable in size and specs, though the Huawei phone’s EMUI operating system and the lack of familiar Google apps (due to the ban on US companies working with the Chinese phone-maker) mean Android fans may prefer Samsung’s. The Huawei foldable is also more expensive, starting at 3,499 euros (about $4,150 today), and may not be compatible with US carriers out of the box.
Read more: Galaxy Z TriFold vs. Huawei Mate XT: One Is the Most Versatile Phone I’ve Ever Used
The Galaxy Z TriFold has a customized Snapdragon 8 Elite chip, the same one that powers last year’s Galaxy S25 series. It won’t feature the latest Snapdragon 8 Elite Gen 5 silicon, which is likely to power this year’s most advanced Android handsets (potentially including the upcoming, but not yet announced, Samsung Galaxy S26 series).
The Galaxy Z TriFold will start at 512GB of storage and packs a 5,600-mAh battery, larger than the Z Fold 7’s 4,400-mAh capacity unit. It recharges at 45 watts, which is typical for Samsung phones, though other premium Android handsets have long ago surpassed that rate, like the OnePlus 15 with 80-watt charging. It has three rear cameras (a 200-megapixel main, a 12-megapixel ultrawide and a 10-megapixel telephoto) and comes in a single color, crafted black.
All told, the Galaxy Z Trifold offers only marginal upgrades over the Galaxy Z Fold 7, and its hardware will likely be surpassed soon when the Galaxy S26 series launches with newer chips.
At $1,000 to $2,000 above other Android phones and foldables, the Z TriFold seems to offer only a single advantage: its massive inner display. While undeniably a technical marvel, that’s not nearly enough added value for most people to justify the steep upsell on your standard smartphone, or even another book-style foldable. For folks who «crave» the most advanced phone on the market, though, maybe it’s worth the expense.
-

 Technologies3 года ago
Technologies3 года agoTech Companies Need to Be Held Accountable for Security, Experts Say
-

 Technologies3 года ago
Technologies3 года agoBest Handheld Game Console in 2023
-

 Technologies3 года ago
Technologies3 года agoTighten Up Your VR Game With the Best Head Straps for Quest 2
-

 Technologies4 года ago
Technologies4 года agoBlack Friday 2021: The best deals on TVs, headphones, kitchenware, and more
-

 Technologies5 лет ago
Technologies5 лет agoGoogle to require vaccinations as Silicon Valley rethinks return-to-office policies
-

 Technologies5 лет ago
Technologies5 лет agoVerum, Wickr and Threema: next generation secured messengers
-

 Technologies4 года ago
Technologies4 года agoOlivia Harlan Dekker for Verum Messenger
-

 Technologies4 года ago
Technologies4 года agoiPhone 13 event: How to watch Apple’s big announcement tomorrow
